The presence of oxygen in the atmosphere of an exoplanet is a sign that life could be occurring. Photosynthetic organisms on Earth absorb carbon dioxide, sunlight and water, and then produce sugars, starches, and energy. The byproduct of this process is oxygen, so if there’s oxygen anywhere else, it will generate excitement.
However, researchers are putting pressure on the notion that oxygen in an exoplanet’s atmosphere means life. If we can exclude other routes that produced the oxygen, it’s not evidence of life.
Scientists can’t rule out these conditions.
Earth is saturated with oxygen. It is found in 46 percent of the crust, about the same amount of the mantle and around 20 percent in the atmosphere.
The Great Oxygenation Event (GOE), which occurred two billion years ago, is the source of oxygen. In ancient cyanobacteria, pigments were developed that absorb sunlight and used it for photosynthesis. The photosynthesis waste product is oxygen, so life has had several billion years to build up oxygen in the atmosphere and crust.
Scientists may find oxygen in exoplanets’ atmospheres, which strongly indicates that life is possible. Simple life could be living in the oceans of the planet, taking in sunlight and releasing oxygen.
However, new research shows that oxygen doesn’t depend on living beings.
The research article is entitled “Abiotic molecular oxygen production—Ionic pathway from sulfur dioxidePublished in Science Advances. The lead author is Måns Wallner, a doctoral student in physics at the University of Gothenburg in Sweden.
Researchers discovered an abiotic source to oxygen, which is sulphur dioxide. Because sulphur is common in celestial bodies and volcanoes pump it into the atmosphere, exoplanets with terrestrial volcanic eruptions could have oxygen in their atmospheres. Life isn’t necessary.
Instead, high-energy star radiation can ionize sulphur dioxide molecules. The formula for sulfur dioxide is SO2It is ionized and the molecule changes. It is now a “double positively charged system.” It then forms a linear structure with the oxygen atoms at one end and the sulfur at the other. This is known as roaming because the oxygen atoms can freely drift in chaotic orbits before settling into new compounds.
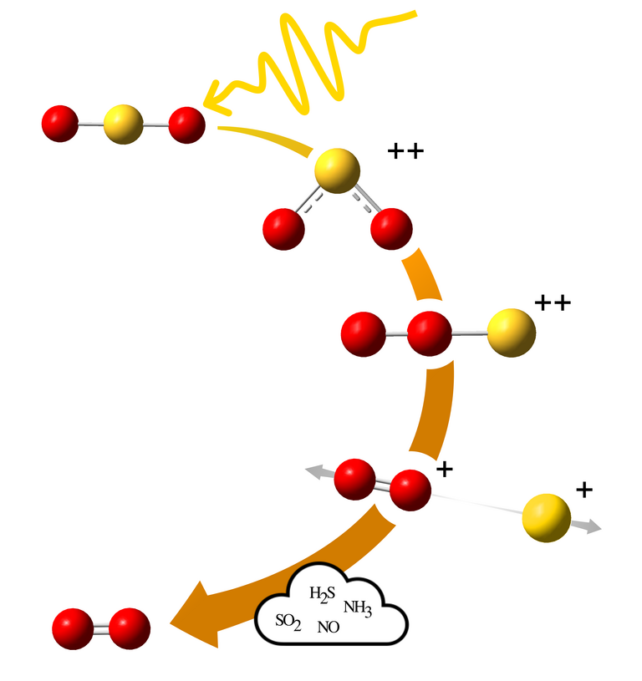
Wallner, the lead author, said that double ionization results in two electrons being released from the molecule. This can cause changes in angle between atoms within the molecule. It was stated in a press release.
“Alternatively, and crucially in this case, roaming may occur. That is, the electrons swap places and the molecule assumes a new form.”
The molecule’s constituents might not change into SO.2 again. The sulphur could be broken up and a simple positively charged oxygen molecule may remain. In this case, the positive charge can then be neutralized by attracting an electron from another molecular. Molecular oxygen (O2) remains, and it’s vital to life on Earth.
This pathway may be responsible for some oxygen found elsewhere. Europa, Ganymede, Io all have oxygen in their atmospheres. Roaming could also be a reason.
Io is a volcanic place – the most volcanic world in the Solar System – so life is ruled out there. Europa and Ganymede have subsurface oceans that could support life. However, this life cannot create an oxygen atmosphere similar to Earth life. To explain the oxygen found on these moons, another explanation is needed.
Researchers believe that this oxygen pathway may also be present on Earth.
“We also suggest that this occurs naturally on Earth,” Raimund Feifel was co-author of this article.
Researchers are investigating the possibility that this ionic oxygen-forming pathway could also work for other molecules. They are interested in finding out if carbon diselenide and other molecules can be subjected to double-ionization.
“We’d like to see if it happens again, or if it was just a coincidence with sulphur dioxide.” Feifel.
Some other researchers have looked at abiotic O.2 sources. Sources Paper 2014Evidence was presented for the production of molecular oxygen from CO2Exposure to high-energy UV radiation.
In a 2015 paperJapanese researchers have shown that near-Ultraviolet light can produce O2Titania (titanium oxide) is used as a catalyst to interact with water on exoplanets.
These findings can help us understand how Earth had an insignificant amount of oxygen before the advent of the GOE. This is because oxygen is so reactive. There must have been a replenishing supply, and these pathways may be responsible.
This research is supported by the James Webb Space Telescope. The telescope is able to study exoplanet atmospherics, one of its science objectives. It has powerful infrared instruments that will reveal the chemical composition of exoplanetatmosphères.
There will be excitement if it finds oxygen. This research shows that oxygen is not only essential for life.
This article was originally published in Universe Today. Please read the Original article.


